News with filters

Strong start to 2026 for Sutter USA
Two intense days in Scottsdale, Arizona, demonstrated just how much energy our network has. With 45 dedicated sales professionals, strong momentum,…

Our first Sustainability Report is here
Taking responsibility – for people, resources, and the future. With our first Sustainability Report for 2024, we show how we strive to live up to this…

All eyes on MEDICA 2025
The MEDICA 2025 is in full swing – and Sutter Medizintechnik is right at the heart of it! On the second day of the trade fair, we’re looking forward…

Sutter USA at the AAO-HNSF 2025
What a weekend it was! The AAO-HNSF 2025 Annual Meeting & OTO EXPO℠ in Indianapolis brought together otolaryngology specialists from more than 70…

First Sutter Young Microsurgeons Award
The Sutter Young Microsurgeon Award was presented for the first time at this year's EANS 2025 in Vienna – an award that recognizes outstanding young…

Strongly connected. Strongly regional.
This week, we had the pleasure of hosting “WRF meets…” at our headquarters in Emmendingen, Germany, in cooperation with the Economic Development…

Apprenticeship Kick-off 2025 at Sutter
On September 1st, the 2025 apprenticeship year officially began – and we’re thrilled to welcome Tom and Alexander as new apprentices in industrial…

We are part of the Robotics Foundation
We are proud to be a founding supporter of the Robotics Foundation at the Work-Life Robotics Institute of Offenburg University, Germany. Together with…

That was Girls' Day 2025 at Sutter
On April 3, 2025, we welcomed Elena, Lia, Medina, and Mila to our company and gave them exciting insights into the profession of industrial mechanic…

Four years at the top: Sutter on Kununu!
Did you also start the new year with a real highlight? We certainly did: Sutter was named a Kununu Top Company for the fourth time in a row in 2025!…

Ready to recharge!
Sustainable travel: Four new electric charging stations are now available on our company premises – powered by 100% green electricity from…

Things run smoothly at Sutter
On June 28, 2024, Emmendingen's city center was transformed into a large running arena: Around 2,600 participants took part in the 14th city run –…

Sutter Distributor Conference (SDC) 2024
Following CEORL-HNS, the 10th Sutter Distributor Conference (SDC) 2024 kicked off in June – this time in beautiful Dublin, Ireland. The focus was on…

We were a gold sponsor at the 59th Youth Researches Competition in Southern Baden
Our commitment to young talent was particularly evident at the South Baden regional competition: as a gold sponsor, we had our own information stand…

Bert Sutter has been voted President of wvib Schwarzwald AG (Association of Industry in Baden e.V.)
We are delighted to announce that Bert Sutter, CEO of Sutter Medizintechnik GmbH, was elected President of wvib Schwarzwald AG on November 10, 2023—a…

Sutter Medizintechnik Wins MobilSiegel 2023!
We are delighted to have received a special award: Sutter Medizintechnik has been honored with the “MobilSiegel – Klimafreundliches Pendeln”…
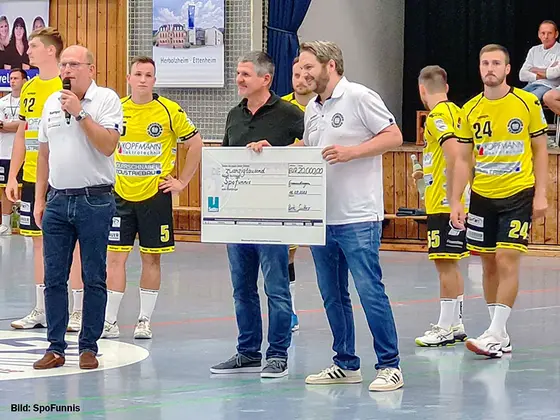
Sutter donates EUR 20,000 to SpoFunnis
At Sutter, we are not only committed to medical progress, but also to social engagement in the local community. That is why we are delighted to…

The EU plans to ban PFASs - what now?
Following the online panel discussion organized by wvib entitled “The EU bans PFAS – what now?”, the Badische Zeitung newspaper took up the topic and…

Focus on PFAS
Not all PFAS are dangerous; they are persistent, and we must prevent their release into the environment. However, not all PFAS are bioaccumulative,…

Sharing knowledge, strengthening trust: EMEA partners visit Sutter
At the end of March, we welcomed ten of our EMEA specialist retail partners to a two-day “Training@Sutter ENT Basic” course at our company…

New application video: „Extensive tumor shrinking with SuperGliss® non-stick ELP Bipolar Forceps“
In our latest surgical video insight, a user from the Department of Neurosurgery at the University Medical Center Freiburg demonstrates the use of the…

…and we did it again! Top Company 2023.
Sutter Medizintechnik has once again been recognized as one of the best employers on kununu. We're proud of this distinction and remain committed to a…

Three Years in a Row – Sutter Named Kununu Top Company Again
We’re proud to be recognized once more as a Kununu Top Company, confirming our commitment to a strong workplace culture and employee satisfaction.

wvib Medical Technology Cluster visits Sutter
In November 2022, the wvib medical technology cluster meeting took place for the first time at the new Sutter headquarters in Emmendingen – a special…

Sutter among top employers of German mid-sized companies 2023
For the sixth consecutive year, FOCUS magazine, in collaboration with its research partner FactField GmbH, has published its list of the best…

A new recruiting campaign for our production facilities
With fresh motifs, new formats, and broad regional coverage, our new recruitment campaign for manufacturing has been launched. The focus is on our own…

Engaging exchange with politics
How do inflation, geopolitical developments, and fragile supply chains affect medium-sized companies in the region? What political course must be set…
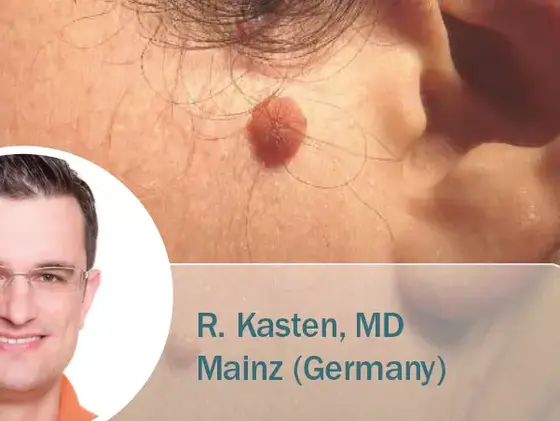
Radiofrequency Ablation of Nevi and Fibroma
Radiofrequency has been established as a gentle surgical method for precise cuts and coagulation in aesthetic surgery. With the impedance-controlled…

Clear visibility in narrow and confined spaces: Application examples using the SuperGliss® non-stick zhora bipolar forceps
With their delicate eccentric tines, the SuperGliss non-stick zhora bipolar forceps are particularly advantageous for surgical procedures in narrow…

Optimising Outcomes in Blepharoplasty Surgery Using Radiofrequency
Ophthalmologist and eye surgeon A. Looi (Ava Eye Clinic, Singapore) reports on her experience with the CURIS® 4 MHz radiofrequency technology in…

Sutter is Kununu Top Company 2022 – and that makes us particularly proud
There are over 3 million companies in Germany – around a third of which are represented on Kununu, the leading platform for employer reviews in…
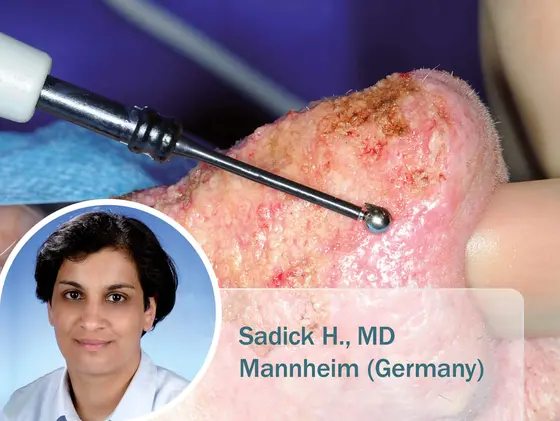
Management of Rhinophyma using 4 MHz Radiofrequency Technology
To date, surgery remains the primary option for the treatment of rhinophyma. ENT surgeon H. Sadick, MD from the University Hospital in…

Socially Committed 2021: Sutter receives the Award for Medium-Sized Companies
Sutter was again awarded the prize for social commitment among small and medium-sized companies. We have received this award by well-known German…








![[Translate to English:] Sutter-Team bei der Siegerehrung des Stadtlaufs Emmendingen [Translate to English:] Das Sutter-Team jubelt bei der Siegerehrung des Emmendinger Stadtlaufs – Einsatz und Teamgeist stehen im Mittelpunkt.](/fileadmin/_processed_/f/6/csm_dsc_2229-edit_dee81a5686.webp)